
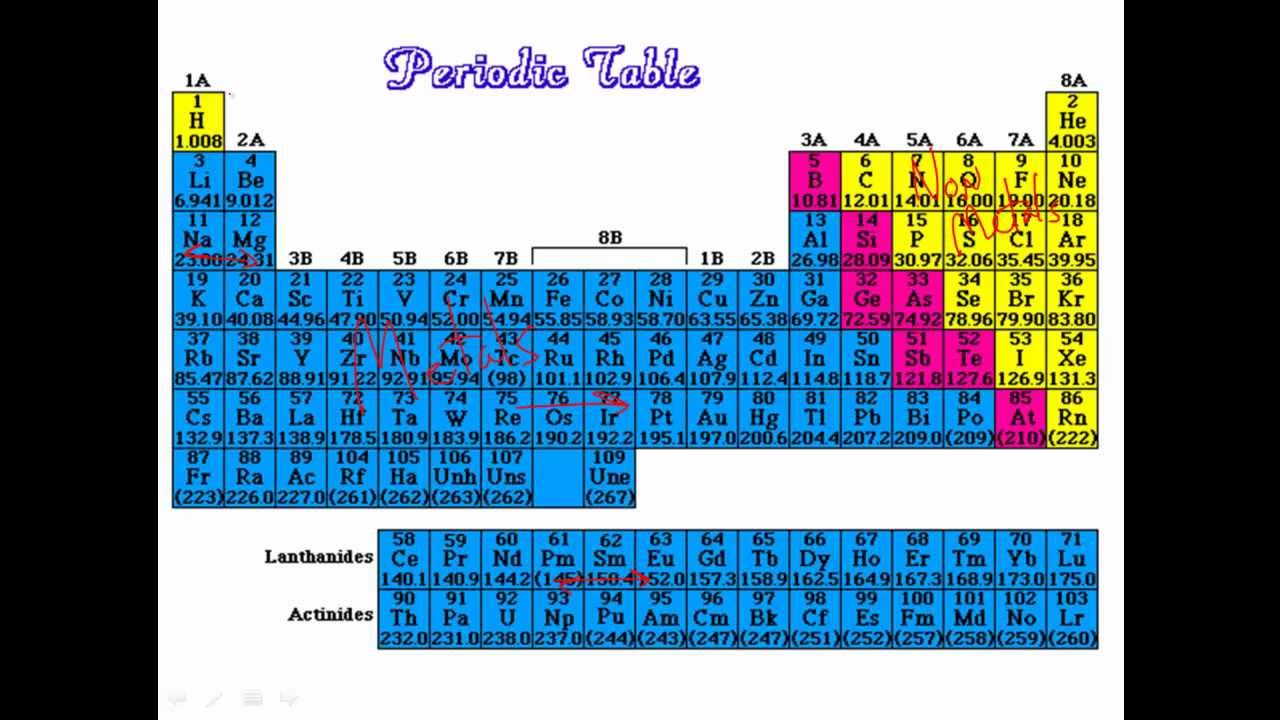
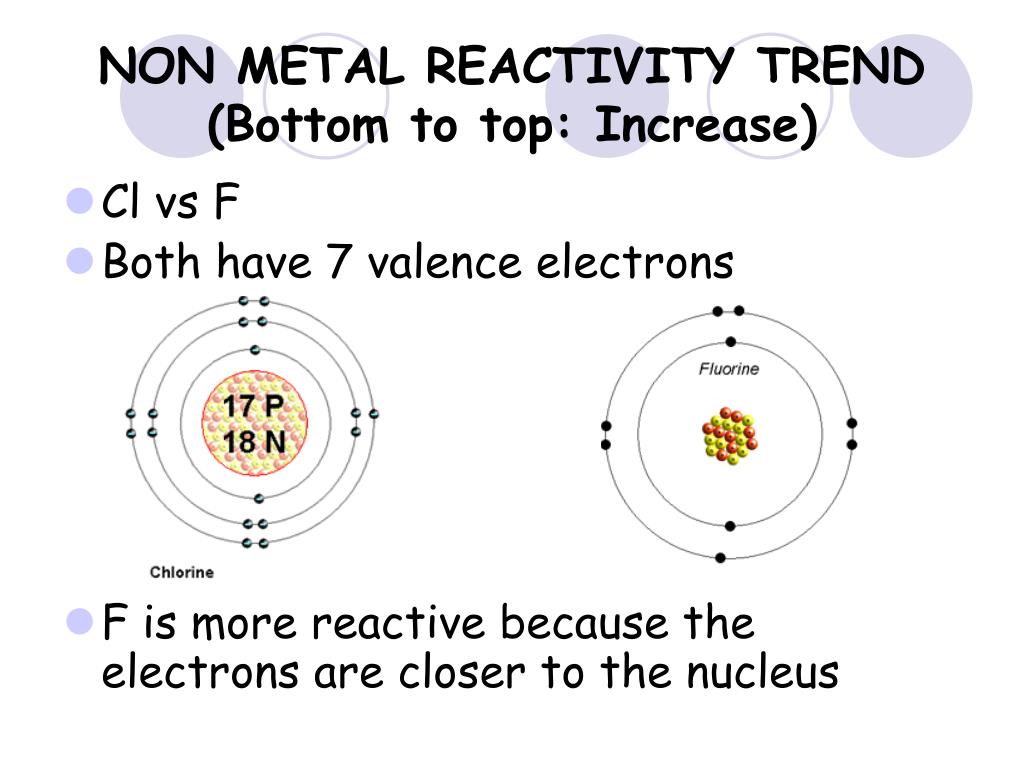
Remind them of what they already know about atoms and elements.Welcome students into class and tell them they will begin a new unit.Before students enter classroom make sure that materials are ready and that the necessary safety equipment is nearby.Non-metals that are up and to the right are closer to the noble gases and do a better job of attracting electrons to their nucleus because they don’t have as many energy levels, and therefore their electrons feel less shielding. For non-metals the trend is that reactivity increases up and to the right. Metals that are down and to the left are closer to the noble gases and have electrons that are easier to remove. The trend for metals is increasing reactivity down and to the left. This also accounts for trends of reactivity. Electrons in higher energy levels are further away from the nucleus and do not feel as much attraction to the nucleus as electrons in lower energy levels. Also as you move down the periodic table the dominant factor is the number of energy levels. Metals react to lose electrons while non-metals react to gain electrons due to their position on the periodic table that corresponds to their valence electrons. This simple idea leads to the reactivity trends we observe. Depending on the number of electrons all ready in their valence shell it may be easier to either gain or lose electrons. Elements all want to be more stable, and the easiest way to accomplish this goal is to either gain or lose electrons to fill their valence shell orbital. Reactivity is a periodic trend that is ultimately related to valence electrons and the process of gaining and losing electrons to become more stable. One major observable periodic trend is reactivity. The development of the periodic table (which occurred well before atomic substructure was understood) was a major advance, as its patterns suggested and led to the identification of additional elements with particular properties. (Most of this text came from the National Science Education Standards Framework draft, pages 89-91). The substructure of atoms determines how they combine and rearrange to form all of the world’s substances.
Today the table’s patterns are now recognized as related to the atom’s outermost electron patterns, which play an important role in explaining chemical reactivity and bond formation, and the periodic table continues to be a useful way to organize this information. Some of these patterns include trends in oxidation numbers, atomic radii, and elemental group/families, just to name a few. As the table is constructed in a logical fashion based off certain properties many other patterns begin to arise, all of which correlate with atomic structure that explains chemical phenomena and interactions among different elements.

Science, 340(6129), 177-180.The periodic table is constructed around the elements and their different characteristics. Direct Measurements of Conformer-Dependent Reactivity of the Criegee Intermediate CH 3CHOO. Structure-dependent reactivity of Criegee intermediates studied with spectroscopic methods. Hydrogen-Bonding Mediated Reactions of Criegee Intermediates in the Gas Phase: Competition between Bimolecular and Termolecular Reactions and the Catalytic Role of Water. An analysis method (Chao et al., 2019) has been proposed to predict the efficiency of water catalysis.Ĭhao, W., Yin, C., Takahashi, K., & Lin, J. (ii) The atmospheric fates of different types of CIs are, thus, different. (i) The reactivity of CIs has strong structure dependence (for example, anti-CH 3CHOO reacts quickly with water but syn-CH 3CHOO does not) (Lin & Chao, 2017). In this talk, an overview of the above CIs will be discussed, including the following issues. Some interesting trends have been found in their reactivity. Kinetics and UV-Vis spectroscopy of a few CIs, including formaldehyde oxide, acetaldehyde oxide, acetone oxide, methyl vinyl ketone oxide and methacrolein oxide have been investigated. A new photolytic preparation of CIs (Taatjes et al., 2013) allows us to direct monitor CIs in real time via its strong UV absorption. Owing to very short lifetime and thus low steady-state concentration of CIs, it is hard to directly detect CIs in ozonolysis experiments. CIs are thought to play roles in atmospheric chemistry, including formation of OH radicals and reaction with SO 2, inorganic and organic acids, water, etc. Ozonolysis of alkenes produces very reactive carbonyl oxides (also called Criegee intermediates, CIs).


 0 kommentar(er)
0 kommentar(er)
